- Home >
- Our Actions >
- Ambassador report
5
Comments
Ozone Layer Depletion |
|---|
|
by Abass Abdullah | 09-09-2018 09:32
|
|
Ozone Depletion
Our atmosphere consists of thin layers of gases, water vapour and particles surrounding the earth. The gases include nitrogen, oxygen, carbon dioxide and helium. Of all the gases, nitrogen has the highest concentration of 78%, followed by oxygen 21% argon 0.93% and carbon dioxide 0.03%. The rest which include neon, helium, ozone, hydrogen, methane, carbon monoxide, krypton, and xenon form the remaining insignificant proportion (about0.04%). The atmosphere may be divided into about five vertically layers namely troposphere, stratosphere, mesosphere, thermosphere, and exosphere. Troposphere is the lowest of the atmosphere. Stratosphere lies above the troposphere up to about 50km. Temperature increase with altitude in this zone. This is called temperature inversion. The rate of decrease is not uniform. At the beginning, temperature remains constant. Then it begins to increase steadily. Stratosphere contains much of the ozone layers. The Ozone absorbs much of the sun?s ultraviolet rays to make the place hot. That is, the sun?s rays heat the place directly and this increases the temperature. The depletion of the ozone layer is caused by the emission of chlorofluorocarbons (CFCs). CFCs composed by chlorine, fluorine, and carbon have a long lifecycle, which favours their accumulation. CFCs do not easily react with other substances. In fact, they break up only through sunlight, which divides their molecules, causing the release of chlorine (Cl). Once the chlorine is released, it is able to react with ozone (O3), to form chlorine monoxide (CIO) and oxygen (O2). Cl +O3= CIO +O2 When the molecules of chlorine monoxide (CIO) meets another molecule of oxygen (O) it breaks up, releasing chlorine (Cl), which can ?destroy? another molecules of ozone (O3), creating the catalytic cycle of chlorine. CIO +O = Cl +O2 The industry production of CFCs started in the 1920?s, causing an average reduction of the ozone layer of 3 per cent. Fortunately, chlorine has ?natural enemies? as well, such as methane (CH4). Thanks to them, the natural ozone layer could recover over 50 years, as long as CFCs are no longer used on a global level. The ozone depletion is also referred to as the ?ozone hole?, due to the fact that its reduction is not uniform, but mainly concentrated in Antarctic region with reductions up to 70 per cent |

|
|
|










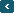 Previous : Sanitation And Cleanliness The...
Previous : Sanitation And Cleanliness The...









5 Comments
Hello Abass, nice to see you again as 21st regional ambassador
Just like what you had written during your 20th regional ambassador term, it's nice to see the statistical record within your report :)
Thanks for citing a very specific outline of the cause and result of the ozone depletion problem!
Posted 14-09-2018 19:47
Wonderful
Posted 12-09-2018 06:14
Hello Abass
With elaborative explanation on how ozone layers are getting holes along with the picture really does help us with our understanding of the cause and process of this problem.
It was most informative!
Thanks for sharing :)
Posted 12-09-2018 00:24
This is a message of hope. We can restore the Ozone layer that has been damaged.
Posted 09-09-2018 18:53
Hi Abass!
It's really fascinating yo know how the stratosphere gains so much temperature to begin with when the troposphere does not. And what I found of new understanding is how the chlorine gas continuously breaks down ozone (O3).
Very informative! And well written indeed.
Thank you!
Posted 09-09-2018 17:51