- Home >
- Our Actions >
- Ambassador report
2
Comments
[February free report] Acid rain fall |
|---|
|
by ALOK DHAKAL | 23-01-2023 01:56
|
|
29th Ambassadorship Title: [February free report] Acid rain fall Month 6, Report 1 Any type of precipitation with high concentrations of nitric and sulfuric acids is referred to as acid rain. Snow, fog, and minuscule pieces of dry material that fall to Earth can likewise represent it. Acid rain typically has a pH between 4.2 and 4.4, whereas normal rain has a slightly acidic pH of 5.6. Sulfur and nitrogen particles that mix with the wet elements of rain are the main contributors to acid rain. The particles of sulphur and nitrogen that mix with water can come from two sources: either man-made emissions from industries or natural events like lightning strikes that release nitrogen oxides and sulfur oxide, respectively, into the atmosphere. Measurement of Acid Rain Below is the chart illustrating the positions of several compounds on the pH scale. A pH scale is used to assess acidity and alkalinity, with 7.0 representing neutrality. A substance's pH scales from 0 to 7, with 0 being the most acidic and greater than 7 being the most basic. A typical rainstorm has a pH of about 5.6, making it mildly acidic due to the dissolution of carbon dioxide (CO2) into water to create weak carbonic acid. The pH of acid rain typically ranges from 4.2 to 4.4. Acid deposition can cause some lakes and streams to become acidic when it is washed into them. In order to gather important data on aquatic ecosystem health and how water bodies react to changes in acid-producing emissions and acid deposition, the Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry is necessary. Effect of Acid Rain Animals, vegetation, and agriculture are all severely harmed by acid rain. All nutrients necessary for plant development and survival are affected. Moreover, acid rain changes the soil’s chemical composition has an impact on agriculture. It affects both people and animals' respiratory systems. The aquatic ecosystem is affected when there is acid rain falls and enters ponds, rivers, and lakes. It results in water pollution and changes the chemical makeup of the water in a way that actually makes it difficult for aquatic ecosystems to exist. In addition to corroding ancient monuments, acid rain also contributes to the leaching of heavy metals like iron, lead, and copper into drinking water. So, we must act now and work for conserving nature. |
 
|
|
|










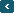 Previous : [Free Report, January, 2023] T...
Previous : [Free Report, January, 2023] T...









2 Comments
Hello, this is your mentor Minkyung.
Thank you for sharing information on acid rainfall. If you provide some statistics related to the status quo and/or the impacts of acid rain, your report would be much richer in content.
For the record, please include the reference to the image you included.
I'll be looking forward to your next report :)
Posted 31-01-2023 23:09
Hi, ALOK DHAKAL!
This is your mentor, Yoon.
Thank you for introducing acid rainfall. Your article is well structured with appropriate subtitles to indicate the contents below.
Great job on writing the free report.
I am looking forward to reading your following report!
Posted 27-01-2023 17:42