No doubt, you've heard the phrase "carbon dioxide is plant food", used as an attempt to counter the idea that carbon dioxide is a pollutant that needs to be regulated and controlled.
It's true that plants use carbon dioxide in their respiration, just as humans use oxygen. However, over the past 800,000 years or so, plants have adapted to conditions with relatively stable concentrations of carbon dioxide in the atmosphere, of about 240 parts per million, give or take about 20 per cent (that is, in a million molecules of air, between 200-280 of those molecules would have been CO2). As the graph below shows, any major changes in CO2 levels during that time took place over thousands, to tens of thousands of years.
The Keeling Curve, showing the past 800,000 years of carbon dioxide concentrations in the atmosphere. Credit: Scripps Institution of Oceanography/UC San Diego
Over the past 150 years, in a relative blink of the eye on the above chart, the burning of fossil fuels - coal, oil and gas - has increased the concentration of carbon dioxide in the atmosphere to around 410 parts per million. That's an increase of nearly 50 per cent over the pre-industrial concentration (280 ppm) and it's a roughly 70 per cent increase over the long-term average set during the past 800,000 years.
If you increased oxygen levels in the atmosphere by those amounts, some things would become easier, but it would not be better for us, overall. Certain types of athletes would find their chosen sport or athletic activity easier, for example. However, the human body is accustomed to a specific range of oxygen levels. Too low and you suffer from hypoxia - too little oxygen is reaching your tissues, especially your brain, and you can die. Too high and your system becomes overloaded, leading to oxygen toxicity, which can cause long-term health issues. There would also be an increase in oxidation (metal objects would rust and corrode faster) and more frequent wildfires (and possibly more fires in general), as it would become easier to spark a flame.
Also, even though plants do benefit from high concentrations of carbon dioxide, they don't tend to benefit in ways that are beneficial to us. For example, plants that we characterize as weeds tend to do the best with higher carbon dioxide concentrations, and while those plants we grow as food may grow larger, the parts of them that we eat have been found to contain fewer nutrients and vitamins. So, while we may get more or larger food plants out of this, we'd have to increase the amount we consume in order to get the same benefits. So it evens out, at best, and certainly does not compensate for all the other negative effects of climate change.
There is also the issue of ocean acidification, which is when the ocean water absorbs carbon dioxide, and it is converted to carbonic acid. This changes the pH of the ocean water, from slightly basic towards neutral, which makes it more difficult for certain basic species to survive.
Bottom line: More of something is not necessarily a good thing in nature, and nature has been "used to" a certain amount of carbon dioxide for close to a million years. Since the excess can and does cause problems in nature (and thus for humans and human civilization), this excess can rightly be considered a pollutant that needs to be limited and cleaned up.
|











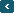 Previous : The Dirtiest River in The Worl...
Previous : The Dirtiest River in The Worl...









2 Comments
Thank you for sharing!
Posted 13-12-2017 19:16
Hi, Ashtha! You've done a really good job in explaining why although carbon dioxide is needed in the atmosphere, too much of it can become a problem. I liked your analogy to oxygen, explaining how although we need oxygen to live and it is very important, too much can be a problem, too. Although I would like to correct you on one part: plants also use oxygen for their respiration! Carbon dioxide is used in photosynthesis, a separate process :) Overall, great job on your report! :D
Posted 01-12-2017 17:44